1s2 2s2 2p6 3s1|Electron Configuration : Tuguegarao To determine the electron configuration of a particular atom, start at the nucleus . Solaire on Anguilla is a wonderful villa experience for those who enjoy a peaceful getaway. My husband and I loved the amazing view of the sea and the island of St Maarten. The sparkling pool and a convenient location to a small, sandy pocket beach makes it easy for lots of water activities. The inside of the villa is beautiful and well maintained.Here is the Kerala Lottery Guessing 4 Digit Number கேரளா லாட்டரி கணிப்பு எண் – இன்று மற்றும் நாளை கடைசி 4 இலக்கம்- கேரளா லாட்டரி
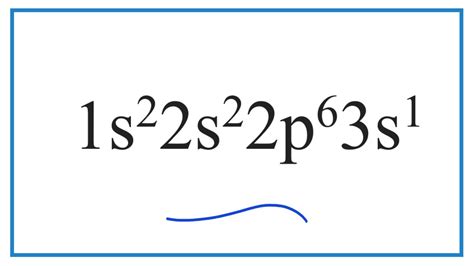
1s2 2s2 2p6 3s1,Learn how to write electron configurations for all elements based on the periodic table. Find examples, rules, and practice problems for different orbital blocks and energy levels.
o figure this out the element with the electron config of we first count the electrons. Those are the small number placed at the top after the letters. For this element we have 2 + 2 + 6 + 1 = 11 .
To determine the electron configuration of a particular atom, start at the nucleus .1s2 2s2 2p6 3s1 Electrons are filled in atomic orbitals as per the order determined by the Aufbau principle, Pauli Exclusion Principle and Hund’s Rule. As per the Aufbau principle the electrons will occupy the orbitals .Learn how to write the electron configurations of atoms and understand the periodic trends in chemistry at Illinois.The electron configuration of sodium can be represented as 1s2 2s2 2p6 3s1. This diagram shows the arrangement of electrons in the atomic orbitals of sodium. The first energy level, represented by the 1s orbital, can hold . A. 1s2 2s2 2p5 3s2. B. 1s2 2s2 2p4 3s1. C. 1s2 2s2 2p6 3s2. D. 1s2 2s2 2p6 3s1. CÂU 2: Nguyên tố X có Z = 17. Số electron ở lớp ngoài cùng của nguyên tố X là: A. 1. B. 5. C. 3. D. 7. CÂU 3: Nguyên tử .Electron Configuration Zinc's full electron configuration is: 1s2 2s2 2p6 3s2 3p64s2 3d10. However, notice that 1s2 2s22p6 3s2 3p6 is the configuration for Argon, a noble gas. Just replace this portion of zinc's electron notation with Argon's chemical symbol in brackets ([Ar].)1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f7 5d1. 78. Platino. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d8. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s1 4f14 5d9. 79. Oro. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d9. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s1 4f14 5d10. 89
Which element has the following ground-state electron configuration? 1s2 2s2 2p6 3s2 A) Na B) Ne C) Al D) Si E) Mg. Like. 0. . option E. An atom of element Na (atomic number 11) contains 11 electrons. The electron configuration of this element is 1s2 2s2 2p6 3s1. Hence, this option. Continue reading. Related Answered Questions. General .1 Example for Na [Ne] 3s1 Na = 1s2 2s2 2p6 3s1 electron configuration neon's electron configuration (1s22s22p6) third energy level [Ne] 3s1 one electron in the s orbital orbital shape Na = 1s2 2s2 2p6 3s1 electron configuration. 2 PRACTICE EXAMPLE Write the longhand and shorthand configurations for: Antimony. 3 .
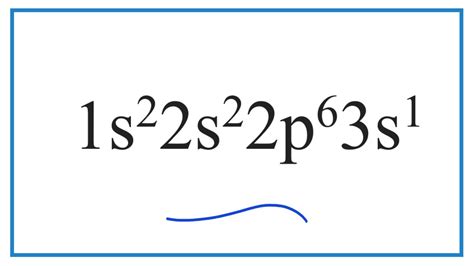
Tem configuração eletrônica 1s2 2s2 2p6 3s2? 1s2 2s2 2p6 3s2 3p6 (K = 2, L = 8, M = 8). Assim sendo, tem-se uma configuração de um íon bivalente. O cobre está localizado no quarto período do grupo 11 (transição externa). Sua configuração eletrônica é a seguinte: 29Cu: 1s2 2s2 2p6 3s2 3p6 4s2 3d9.1s2 2s2 2p6 3s1 Electron Configuration Tem configuração eletrônica 1s2 2s2 2p6 3s2? 1s2 2s2 2p6 3s2 3p6 (K = 2, L = 8, M = 8). Assim sendo, tem-se uma configuração de um íon bivalente. O cobre está localizado no quarto período do grupo 11 (transição externa). Sua configuração eletrônica é a seguinte: 29Cu: 1s2 2s2 2p6 3s2 3p6 4s2 3d9.1s2 2s2 2p6 3s11s2 2p6 3-12 13 22s 2P Al 2 2s2 2p6 1s2 1s2 Si 2 2p6 Is 1s2 22p6 1s2 1s2 2p6 1s22s 1 s2 2s2 2p6 Bloc s Is Is Is 1s22s 2P 1 2s2 2p6 3s 3p 3s 3p 3s 3p Bloc p 3s 3p 3s 3p Tableau périodique restreint aux 18 premiers éléments chimiques. Tableau périodique : une organisation structurée Doc. 4 des configurations électroniques
The electronic configuration 1s2,2s2, 2p6, 3s1 and 3p1 is correctly describe by (A) The excited state of Mg+ (B) The excited state of Na+ (C) The exci1s2 2s2 2p6. Sód Na: 1s2 2s2 2p6 3s1. Magnez Mg: 1s2 2s2 2p6 3s2. Glin Al: 1s2 2s2 2p6 3s2 3p1. Krzem Si: 1s2 2s2 2p6 3s2 3p2. Fosfor P: 1s2 2s2 2p6 3s2 3p3. Siarka S: 1s2 2s2 2p6 3s2 3p4. Chlor Cl: 1s2 2s2 2p6 3s2 3p5. Argon Ar: 1s2 2s2 2p6 3s2 3p6. Potas K: 1s2 2s2 2p6 3s2 3p6 4s1. Wapń Ca: 1s2 2s2 2p6 3s2 3p6 4s2. Skand Sc: 1s2 .1s2 2s2 2p6 3s1. Electrons are filled in atomic orbitals as per the order determined by the Aufbau principle, Pauli Exclusion Principle and Hund’s Rule. As per the Aufbau principle the electrons will occupy the orbitals . Haz clic aquí 👆 para obtener una respuesta a tu pregunta ️ Elemento cuya distribución electrónica es 1s2 2s2 2p6 3s1 Angelamavp2706 Angelamavp2706 25.03.2020 N ⇒ 1s2 2s2 2p6 3s1 M ⇒ 1s2 2s2 2p6 3s2 3p5. kimheesook100820 kimheesook100820 28.08.2020 Química Universidad contestada ¿Cuántos electrones transferidos hay al unirse los siguientes elementos? N ⇒ 1s2 2s2 2p6 3s1 M ⇒ 1s2 2s2 2p6 3s2 3p5 Ver respuesta Publicidad
Consider the following electron configurations to answer the question: (i) 1s2 2s2 2p6 3s1 (ii) 1s2 2s2 2p6 3s2 (iii) 1s2 2s2 2p6 3s2 3p1 (iv) 1s2 2s2 2p6 3s2 3p4 (v) 1s2 2s2 2p6 3s2 3p5 The electron configuration that belongs to the atom with the lowest second ionization energy is _____. a. v. b. iv. c. iii. d. ii. e. i
The electron configuration of sodium using standard notation is: 1s2 2s2 2p6 3s1. Sodium has 11 electrons, and the first two fill the 1s sub-shell. The next two fill the 2s sub-shell, and the remaining seven electrons fill the 2p and 3s sub-shells. . Its configuration is 1s2 2s2 2p6 3s2 3p6 4s1 3d10. In summary, when ions form, electrons can .
Bb:1s2 2s1; Cc: 1s2 2s2 2p2; Dd:1s2 2s2 2p4; Ee: 1s2 2s2 2p5; Ff: 1s2 2s2 2p6; Gg:1s2 2s2 2p6 3s1; Analise as afirmativas a seguir. ( ) Apenas dois desses elementos apresentam configuração eletrônica de gás nobre. ( )Aa e Dd podem formar moléculas diatômicas homonucleares.
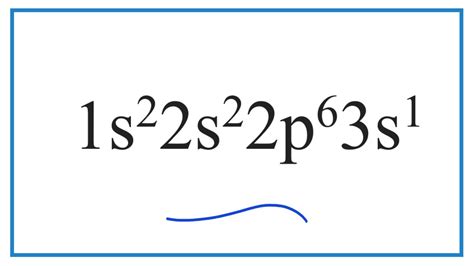
(Unesp-SP) Os elementos X, Y e Z apresentam a seguinte configuração eletrônica: X: 1s2 2s2 2p6 3s1 Y: 1s2 2s2 2p6 3s2 3p4 Z: 1s2 2s2 2p5 Assinale a alternativa correta: a) X forma cátions de carga + 2. b) Y possui maior eletronegatividade do que Z. c) X possui energia de ionização maior que Z. d) Z é halogênio.
1s2 2s2 2p6 3s1|Electron Configuration
PH0 · Which element has the electron configuration of 1s2 2s2 2p6 3s1
PH1 · What is 1s^(2)2s^(2)2p^(6)3s^1 the electron configuration for
PH2 · What element has the electron configuration 1s2 2s2 2p6 3s2 3p2
PH3 · The Electron Configurations of Atoms
PH4 · Na
PH5 · Electron Configuration Calculator
PH6 · Electron Configuration